Potential of Hydrogen
Section 4.1
(Published Feb.10, 2024; rev.250213)
| 🇨🇦 | 🕆 | 🇨🇦 | 🕆 | 🇨🇦 | 🕆 | L🇨🇦 |
The pH scale is arguably the most underrated topic as it pertains to health and longevity. I’ve discovered that in certain disciplines and fields of interest, pH is considered very important and studied intently. Whereas in others, pH is deemed not important or perhaps even irrelevant. Hopefully I can pique your interest and bring its relevancy back to the forefront.
From my opinion, appropriate pH levels are just as important for our cell's environment, as atmospheric-oxygen levels are for humans.
Full Disclosure Statement: Please be well aware that I am not a health professional. The information herein is based upon personal life-experiences, research, and knowledge of certain topics which the Lord has graciously guided me through.
Take-note that unique information herein (of which there’s a substantial part) has yet to be evaluated by the health-care industry. As always, heed the advice of your physician and ask for their advice, before varying either diet or lifestyle.
We understand that the human body is comprised of trillions of cells and is quite complex. A little common sense here, reminds us of the fact that we need a certain length of time to change the course of one's health. Not to mention, our daily habits.
Lawrention
4.1.1 Potential of Hydrogen (pH) Definition
Depending upon your preference, pH is short for either potential of hydrogen, or power of hydrogen. It’s a standard scale of the molar concentration (chemical term) of hydrogen-ions within aqueous fluids. The pH scale represents an aqueous-solution’s ionic gradient, which can be simplistically stated as being either acidic, alkaline, or neutral.
The pH scale ranges anywhere from zero thru fourteen, whereby seven is neutral. Pure-water has a neutral pH, so it has a value of seven.
The group of rising values on this pH-scale from zero to just below seven - is referred to as acidic; in contrast, anything above seven on the pH-scale is referred to as being alkaline.
4.1.2 Potential of Hydrogen Relevance
The following are examples where the pH scale is highly relevant.
- Potable Water. Water supplied to us from treatment facilities needs to be highly alkaline, so lead and other heavy metals won’t leach out of pipes and get distributed through tap water.
- Our GI-tract relies upon secreted enzymes having specific pH levels in order to regulate the digestive process.
- Appropriate pH levels are crucial for human reproduction. In males, an appropriate systemic pH is required to create healthy quantities of sperm. In females, pH levels in the womb determine the sperm’s response and motility. I surmise that the wombs pH-gradient is how sperm determines the egg's location.
- Our circulatory blood is held within a narrow-band of pH (7.35 - 7.45). If its pH climbs too high, the medical condition for this is referred to as alkalemia. If it gets too low, the condition is referred to as acidemia. Either of these conditions are quite serious and can be life threatening.
- Food and beverage industries use pH values to ensure the quality of their product; both during and after processing activities. For example, the souring of milk can be electronically sensed via declining pH levels.
- Gout is a form of arthritis and is a sign of the blood being overly acidic. An over consumption of alcohol, soda, meat, and seafood, all contribute to its condition. Often-times the onset of gout isn't noticed until pain develops in one of the big toes.
- PH levels are studied to determine the success or failure of medical implants. These implants include those like: artificial hips, knees, screws and rods for bone repairs, pacemakers, and coronary stents. Both the success and lifespan of these implants is often determined by how well they withstand acids from local blood and tissue.
- Hair shampoos, conditioners, dyes, and treatments, can all skew the natural pH balance of one’s hair. Its texture (curly, wavy, straight) may change as its pH changes.
- There is ongoing research regarding the role of pH for the healing of wounds and creating biofilms. It appears like fresh wounds have an alkaline pH and as they begin to heal, become more acidic.
4.1.3 pH Within Aqueous Solutions
This concept of pH is only relevant for aqueous (water-based) solutions. The reason for this, is due to water’s inherent ionic characteristic. Water molecules continually share protons and electrons amongst themselves, through ionic activity. This self-ionization feature makes water ideal as both a solvent and an environment to house living organisms.
Through ionic activities, as the ratio of (momentarily) free protons and free electrons within an aqueous-solution changes, its pH level changes. Simply put, pH is the ratio of free-protons versus free-electrons within an aqueous solution.
This self-ionization characteristic of water, is why water molecules (H2O) cannot be significantly compressed. Even to the depths of the sea, its chemical properties remain the same.
Consider the formation of the earth, where tremendous heat and pressure changed the molecular structure of soil and rocks; and yet.... the chemical-structure of oceanic-water at tremendous depths remains constant.
Examples of aqueous fluids in which pH measurements are relied upon are: salt water, blood plasma, saliva, urine, interstitial fluid (intercellular fluid), and vinegar.
4.1.4 The Scientific Definition of pH
I’ve described that pH values basically represent a ratio of free electrons verses free protons. As a fundamental concept this is true; however, for those who are scientifically inclined - I need to qualify this point further.
Firstly, ~ 99.99% of all hydrogen atoms on earth, have one proton, one electron, and no neutrons. This common isotope of hydrogen is referred to as protium. Hydrogen (H) and more precisely protium (1H), is quite often used for scientific-modelling purposes, since it’s the simplest of all chemical elements.
Protons carry the elementary charge of +1, and electrons carry the opposing charge of -1. Since these two particles have opposing electrical charges, they (typically) attract each other, thereby creating a hydrogen atom.
This concept of pH, teeters between the two sciences of chemistry and physics.
A free proton that's viewed through the lens of physics, and a positively-charged ion that's viewed through the lens of chemistry - are basically the same thing. These two scientific fields often use different terminology to describe the same phenomenon. Each are merely looking at it from two different perspectives.
Free protons are always portrayed as having an elementary charge of +1, and are basically an ion.
Within the chemistry realm, a (momentarily) free proton is referred to as a positively-charged hydrogen-ion (H+). Here, it’s assumed that a hydrogen atom has been momentarily stripped of its associated electron. What remains of the atom, is a positively-charged hydrogen ion.
Since hydrogen ions have a positive elementary-charge, they can optionally be referred to as cations. Cations are positive ions, whereas anions are negative ions.
Within the science of physics, a proton may be studied as a free particle, and thereby disassociated from any atomic structure. Hence, a proton doesn’t necessarily have to be always associated with either a chemical-reaction or an electron. In physics, the proton is often considered to be a lone particle, and holds an elementary charge of +1.
The pH scale is logarithmic, and the difference between any two whole-numbers on its scale represent a tenfold difference of ionic activity. The density of hydrogen ions occurs over a tremendous range in order-of-magnitude. Therefore, a very slight change in any pH measurement, correlates to a tremendous variation of hydrogen-ions.
The pH scale is inverse. At first glance, since pH is based upon the density of hydrogen ions it seems backwards; as the 0 thru 14 numbered scale is inversely proportional to the density of hydrogen ions. Why is this so?
This holds true, because it’s the base-10 exponent which is used for the scale itself. The actual equation is this: pH = -log [H+]. To clarify, a few examples are shown below.
- The pH value of 2 represents: 0.01 mole of hydrogen-ions per liter (mole x 10-2).
- The pH value of 8 represents: 0.00000001 mole of hydrogen- ions per liter (mole x 10-8).
- The pH value of 14 represents: 0.00000000000001 mole of hydrogen-ions per liter (mole x 10-14).
As we see, it’s the base-ten exponent from these prior examples (2,8,14), that’s used to number the pH scale.
Further, from a chemistry standpoint - this concept of pH can also be described like that of the following.
Since water molecules (H2O) momentarily separate during ionic activity, water can be thought of as being a mix of H+ (hydrogen) and OH- (hydroxide) ions.
Therefore, a solution of neutral pH is observed when the quantities of both H+ and OH- ions are equal. Hence, as an aqueous solution becomes more acidic… there are more H+ (hydrogen) ions than OH- (hydroxide) ions. Vice versa is true as well. When the solution moves towards the alkaline side, there are more OH- ions and less H+ ions.
As for myself, I like to keep things as simple as possible. Since ionic activity cannot occur without (momentarily) having free electrons and free protons, I conceptualize pH in the following manner. PH is a ratio of free protons verses free electrons within an aqueous solution. Everything else within this solution (of atomic structures), can be considered as electrically-neutral from a pH perspective.
4.1.5 pH Reference Values
As far as I can tell... any government-regulations in North America, regarding the disclosure of pH-values for food and drink products that were prepared for human consumption - are nil to none. As a population, we are basically left on our own as it relates to pH-values for food and drink.
For comparisons sake, I've created a few diagrams which show pH values for some of our common consumables. Note that the pH values which follow, were compiled from various sources, so some values may be considered as not being exact.
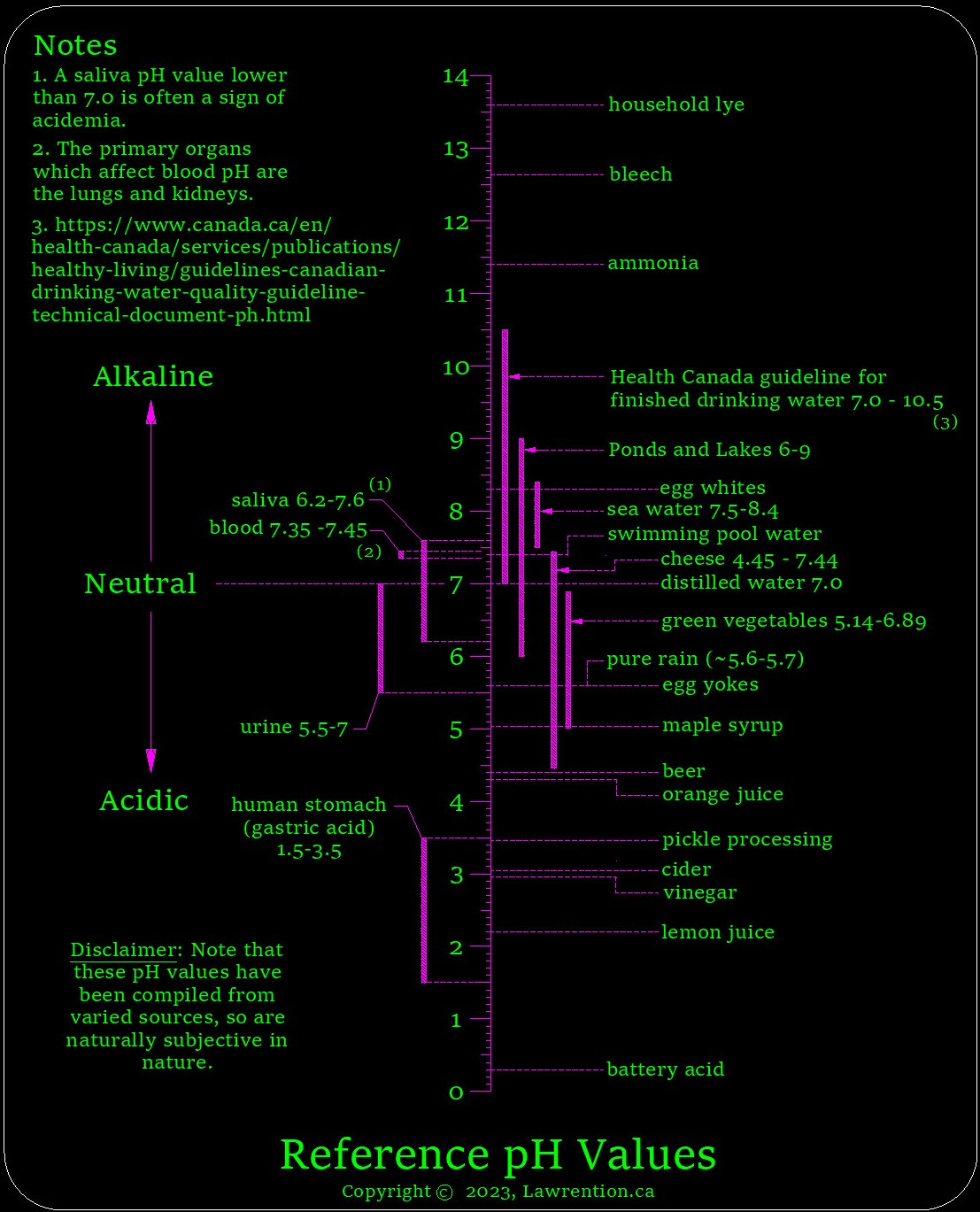
Points to consider from the prior graphic.
- Distilled-water falls directly in the center of the pH scale, and has a neutral pH.
- Note the hierarchy of bodily fluids, which are on the left side of the scale. The blood comprises a narrow alkaline-band of pH, 7.35 to 7.45.
- Saliva, has a band of pH which straddles the neutral value of 7.0.
- The range of pH for green vegetables, goes from 5.14 to 6.89, and is located on the weaker side of the acidic-end of the scale.
- There's great news here if you like cheese. Although cheese has an overall wider pH-band than the greens, it's midpoint is about the same, so should be quite healthy. If I recall correctly, I believe that it's the aged cheeses which are the most acidic.
* Note on pH which was added in January 2025. I've found that Robert O. Young who is a doctor as well as a scientist, and one who has spent a great deal of time studying the body's pH and blood - earlier stated that an optimal saliva and urine pH should both be 7.2. A link to his profile can be found here. An optimal pH of 7.2 for urine is somewhat surprising to myself.
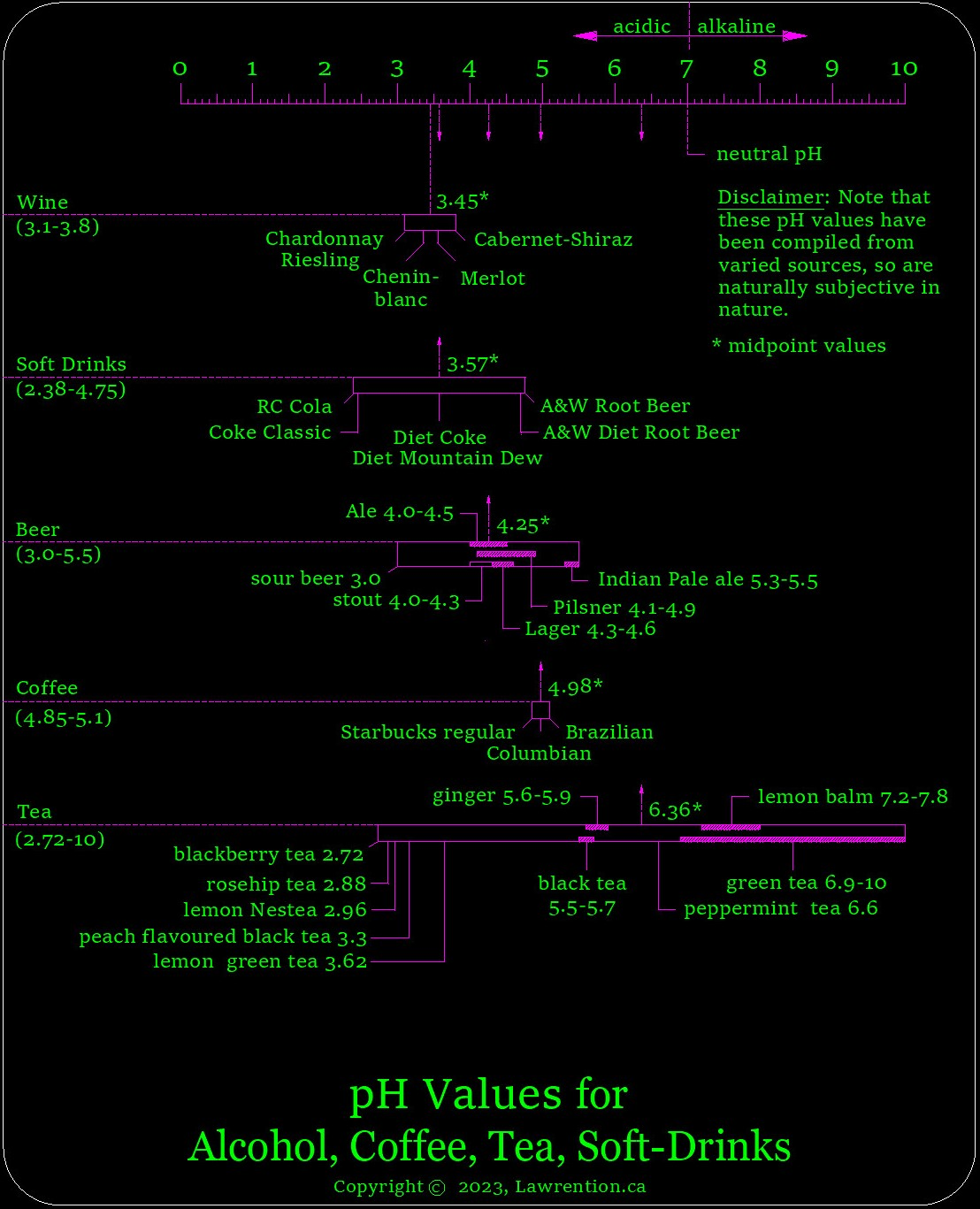
Points to consider from the prior graphic.
- Using midpoint values of certain pH groups, and beginning with the most acidic... the order of pH is as follows: wine (3.45), soft-drinks (3.57), beer (4.25), coffee (4.98), tea (6.36).
- The good news here, is that the tea, beer, and soft-drink categories - all have quite a wide variation of pH to choose from. Hence, there's likely a less-acidic product available than what you've been accustomed to using.
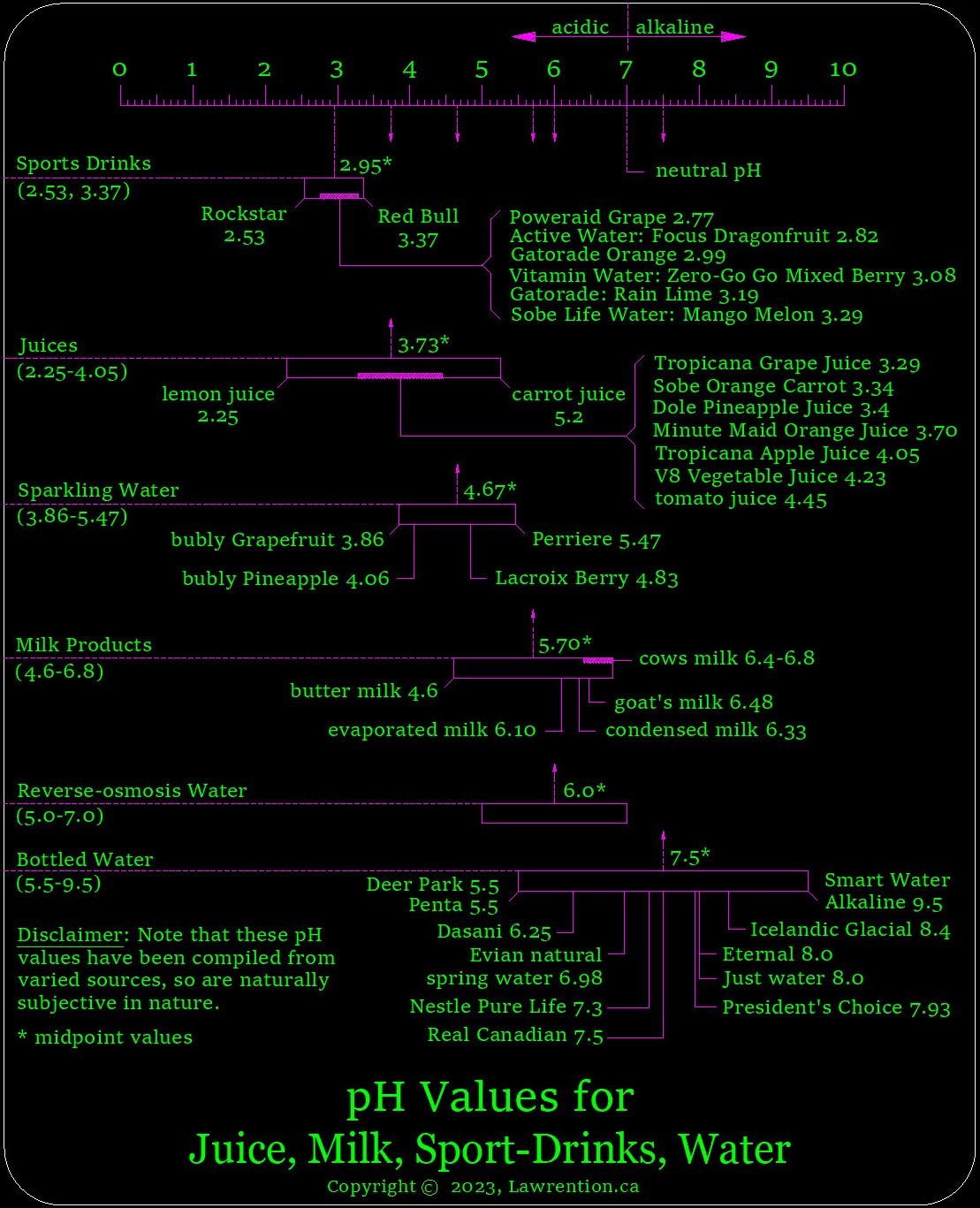
Points to consider from the prior graphic.
- Sports drinks are quite acidic, and generally even more acidic than wine, while comparing each group as a whole.
- Reverse-osmosis water is more acidic than a good number of bottled-water brands.
- There is quite a wide range of pH for bottled water and many brands to choose from. While pH is important, it's obviously not the only criteria which distinguishes water quality.

Since pH values are typically not shown on processed drinks, you might want to check their pH with your own pH-meter. Many of these meters are no longer expensive. The pH meter that I use is the GuDoQi (box-model EZ-9909SP) from Amazon. The primary precautions for its use, is merely ensure that the tip is always clean to prevent cross-contamination and maintain its accuracy. Simply rinsing the tip with water immediately after its use usually does the trick. A small commission is automatically returned to this site if there's a purchase of the product.
4.1.6 pH from a Biblical Perspective
This concept of pH and having a yin and yang of matter, takes us to the creation-process within the book of Genesis.
Genesis chapter-one, explains to us that there were originally three fundamental categories of matter used for creation; light, darkness, and water. These were the building blocks of God's creation process. I consider that these three categories are not only how we perceive them with our senses, but more fundamental in nature as well - like particles.
The light and dark categories are the yin and yang of matter; whereas, water is a mix of both light and darkness. For our discussion at hand, please refer to the following chart.
Fundamental Categories of Matter
|
Category |
Type of Matter |
Characteristics |
|
Light |
electron particle |
Stable particles, comparatively light in weight when compared to protons. |
|
Dark |
proton particle |
Stable particles, comparatively heavy as compared to electrons. |
|
Water |
Water-like, either chemical-wise (H2O) or primordial-wise. A mix of both light and dark particles. |
A self-ionizing mix of both light and heavy particles. A stable solvent. |
In the book of Genesis, I can only surmise that the primordial “water” which God began with, may have been different than the water we’re accustomed to. I'm suggesting this, only since water as we currently understand it - is a chemical compound. Early within the creation process, I surmise that there had to of been something more basic in nature.
I believe that the matter which was initially referred to as “water” was referred as such, due to its self-ionizing feature. In other words, it was a solvent, could not be easily compressed, and was relatively stable. Perhaps it was later in the creation process when God referred to water as “seas” (Gen.1:9), that this water held the chemical properties which we now take for granted as H2O.
I suppose that I should qualify the “dark” category from the above chart. Although somewhat related, what I’m discussing here is not the “hypothetical dark-matter” which cosmologists speak of. What I’m referring to, stems from a biblical standpoint and out of the two fundamental categories of matter.... light and dark. All of the physical matter around us, in which we can see and touch - falls under these two categories.
The yin and yang of particles within atomic structures, are the electrons and protons. Minimally, all atomic structures contain these two basic particles. Optionally, atomic structures can also contain neutrons. In the prior chart, these yin and yang particles are placed into their respective categories of “light” and “dark”.
Under the umbrella of physics, both electrons and protons are classified as being “stable” particles. So, regardless of the fact that there are a considerable amount of particles within the standard-model of physics...... only a very-few are classified as stable. The remaining, within the standard model - all decay quite rapidly.
From a biblical perspective, the neutron particles are an aggregate mix of both the light and dark categories of matter that I've described herein.
It’s been experimentally established, that when neutrons are left to their own demise.......... they decay in approximately fifteen minutes. Hence, neutrons are not stable once they are freed from their respective atomic-structure.
Note that all atomic structures (both chemical elements and molecules) are comprised of merely electrons, protons, and optionally - neutrons.
Electrons and protons are on the opposite side of the fence as it relates to heat dissipation. Since the mass of protons are 1,836 times more than electrons, it’s the comparatively massive protons which retain much of the heat during atomic activity.
It is the “orbiting” electron (or electrons) which fan an atom’s nucleus which cools it down. To explain this cooling phenomenon further, there has to be a finer medium of particles in order to dissipate the heat. Therefore, we need to go down another “level” into a mysterious sea of sub-sub (sub2) atomic particles. Since going down this rabbit-hole will distract us from the matter at hand, we will forego this topic for the time being.
The heat differential (mass effect) between electrons and protons, is predominantly why our stomach is acidic and not alkaline. It’s the mass of free protons (hydrogen ions) that heats the liquid and food-stuffs within our stomach, and facilitates digestion.
As a basic principle: within an aqueous-fluid medium (solvent), as its pH level becomes lower (more acidic) its temperature tends to rise, and as its pH level gets higher (more alkaline) its temperature tends to fall.
In the Bible it states that there is no darkness within God; none! (1st-John, 1:5). In contrast, our human bodies were created to be a mix of both light and darkness.
Setting the spiritual aspect aside for a moment, this concept of light and dark - is comparable to electrons versus protons within physics; and alkaline versus acidic (or base versus acidic) within biology. If our body gets too far out of balance one way or another, we become unhealthy and are prone to disease.
As a single organism, our bodies are a ratio between the yin and yang of matter - electrons (light) and protons (dark).
The neutron particles within each of our bodies, are a mix of both light and dark. Note that under the study of pH, neutrons are a non-factor.
This is so, because neutrons remain inside their respective atomic-structure, and within the nucleus. Therefore, they do not take part in ionic activities which occur around perimeters of atomic structures.
Our body has a “normal” range of pH for blood, urine, saliva, stomach acids, etc. Since all of our food and drink is comprised of merely three basic particles (electrons, protons, neutrons), and similarly our human body as well.… whatever we eat and drink, has the potential to change our body's ratio between the two fundamental "light" and "dark" categories of matter.
It’s no accident that we were prescribed to eat greens from our creator (Genesis 1:30 and 9:3). Green vegetables are on the mildly-acidic portion of the pH scale.
Recall that Adam and Eve were tempted with an apple which is highly acidic, and not with asparagus and broccoli.
The colour green is the midpoint of the visible-light spectrum; whereas, red and blue are on opposing sides. So, just due to the colour itself - green fruit and vegetables are basically mediators between the various coloured foods during digestion.
As we begin to pull these facts together, it’s no wonder that greens are a powerhouse of nutrition.
4.1.7 pH Balance of Bodily Fluids
Our body is continuously working to achieve balance between the "light" and "dark" fundamental categories of matter. Although the pH of blood is likely the most crucial, since blood delivers oxygen and nutrients throughout our body... all bodily fluids are trying to strike a balance.
The pH of our blood is heavily influenced by what occurs within our lungs and kidneys. I have heard of instances where the blood can become too alkaline, but I believe this is more the exception than the rule. There are often underlying health issues whenever the blood is too alkaline; however, it does occur.
If the pH of our circulatory blood climbs too high, this condition is referred to as alkalemia. If it gets too low, this condition is referred to as acidemia. Either one of these conditions is quite serious, and can result in death.
Note, that I'm not providing a list of issues here, which could lead to either alkalemia or acidemia. For our purposes herein, we are more interested in long-term chronic conditions (not serious diseases) that may be possible to correct through a change of diet.
We have over 30 trillion cells within our body, and appropriate pH levels within each cell are crucial. The (average) pH of a cell's cytosol is slightly above neutral pH. Abnormal cell pH is indicative of disease.
Further, our saliva's pH usually falls within the ~6.2 to 7.6 range, and our urine's pH between ~5.5 and 7.0.
Although I believe that our bodies were designed to be very close to net-neutral pH; each of our bodily fluids, has a unique pH level in which they attempt to adhere to. Moreover, the body itself establishes a hierarchy of bodily-fluid pH, where some pockets of fluids are prioritized above the others.
It stands to reason, that likely the pH of systemic blood is extremely high on the priority list; whereas, the pH of urine is quite low on the list. So, during a period of poor nutrition, the body has to make a decision as to which pH levels to maintain and which ones to sacrifice.
In addition, note that the average cell-pH is neutral or slightly above neutral. Individual cells have varying pockets of pH within, regardless of what the body does as a whole. Since cells get most of their resources from the bloodstream (either directly or indirectly), systemically - the body should (or will likely) prioritize the consistency of blood-pH above all else.
4.1.8 Food-Cravings are Heavily Based Upon pH
Our body naturally craves food and drink which is more acidic than alkaline. This includes consumables like coffee, alcohol, meat, sweets, acidic fruit juice, and junk food. All of these items heavily lean towards the acidic-side of the scale.
In contrast, we normally don’t crave food and drink which sway towards the neutral or alkaline side of pH. Examples of such are: almonds, asparagus, buckwheat, cauliflower, fresh coconut, eggplant, ginger, green tea, green vegetables, lentils, milk, onions, peppermint tea, quinoa, squash, tofu, water. Now, although the odd person may prefer an item or two from this prior list over an acidic food, the foods on this list are certainly not addictive.
Our creator understood that humans naturally crave the dark side of nutrition, which is why we were instructed to eat greens in the first place. Since animals can’t read, I'm sure this instruction was directed more towards us than them. Greens are here on earth to balance out our diet and we require them daily.
Merely by living in a highly-industrialized society, our bodies are more prone to being acidic verses alkaline, and we are far more likely to be on the "dark" side of the nutritional equation than our ancestors.
While considering the body as a single unit (organism), I believe that most of us teeter towards the acidic side of pH rather than alkaline. Hence, it’s the electrons which our body is being continually short-changed.
4.1.9 Antacids
Since stomach issues are often the result of ingesting foods and drink which are on the acidic side of the pH scale, I've inserted this subtopic of antacids.
Due to stomach problems like acid reflux and heartburn, people often reach for an over-the-counter antacid medication. For chronic heartburn, gastroesophageal reflux disease (GERD), or ulcers; some people use prescription proton-pump inhibitors (PPIs) to reduce stomach acid. Examples of these drugs are shown below, and like all drugs they can potentially have side effects.
- lansoprazole (Prevacid)
- omeprazole (Losec, Prilosec)
- pantoprazole (Pantoloc, Protonix)
Consider that chronic use of antacids or proton pump inhibitors, can inevitably lead to the opposite problem. This is a condition referred to as hypochlorhydria, and occurs when the stomach’s acid-secreting glands merely stop working. As a consequence of this, the stomach has a difficult time digesting certain foods. This is not only uncomfortable, but may also lead to infections and nutritional deficiencies.
The silver lining here, is if no permanent damage has occurred to the GI tract; then merely by switching to more of an alkaline-based diet and given adequate time…. the painful and uncomfortable symptoms will likely diminish, or perhaps even disappear altogether.
4.1.10 Potential of Hydrogen Summary
Our body is normally considered to be electrically-neutral as a whole (net neutral). It's the energetic ions which sway our bodily fluids towards either the light side or dark side of the two fundamental categories of matter.
If you have a difficult time understanding all this, please don’t get bogged down by the lingo. Just understand that all matter as we (currently) comprehend it, is biblically classified as being either light, dark, or a mix between the two. The following chart compares the two sides.
|
Matter Biased Towards the Light Side. |
Matter Biased Towards the Dark Side. |
|
Electrons (electrically negative) |
Protons (electrically positive) |
|
Anions (negative ions) |
Cations (positive ions) |
|
alkaline pH solution |
acidic pH solution |
Examples of Blended Matter (both light and dark)
neutron particles
chemical elements
simple molecules
compound molecules
The aqueous pH environment is an energetic one to say the least. It's a medium for chemical reactions, whereby certain reactions will rise to the occasion only during a specific level of pH.
Ionic activity sets the stage for molecular interactions. It pits certain strains of molecules against others, and their reaction rate relies upon the solution's pH. Each molecular strain prefers a certain frequency of ionic interaction. Otherwise, molecular strains will remain non-reactive and dormant; regardless of the fact that they're in the midst of a chaotic sea of ionic activity.
The species of molecules which dominate chemical-reaction-rates within a specific pH-solution (e.g; a pH of 5.4), are those which strike an appropriate energy balance, and thereby achieve a continual series of reactions.
NEXT: 4.2 Electrolytes and the Acid/Base Balance
Health and Longevity ---> Contents
Revision 250204